The Genomics Core Facility (GCF) is equipped with two liquid
handling robots, a MultiProbe II and a Biomek FX. The robots
are operated by GCF personnel on a fee for service basis.
The Perkin Elmer MultiProbe II EX is an eight-tip
instrument designed to pipet 2ul up to 250ul volumes into 96
and 384 well plates, Eppendorf tubes, and other labware.
This robot is used routinely to pipet 384 well RT-PCR plates
with a 10ul total reaction volume. When the cost of supplies,
reagents, and technician time is calculated, robotic pipetting
is the most accurate and cost effective answer for the pipetting
of 384 well RT-PCR plates. GCF will schedule up to six 384 well
plates for robotic pipetting in one day or the equivalent of
twenty-four 96 well plates enabling experiment data to be
available to the researcher rapidly. The MultiProbe’s
configuration gives a high degree of programming flexibility.
Custom pipetting programs can be written for special projects.
Please contact GCF personnel for project consultation well in
advance of your planned start date.
The Beckman Coulter Biomek FX is available for custom programming
of high through-put pipetting tasks. It is configured with a 96
tip head which will make pipetting transfers and liquid additions
to and from 96 and 384 well formatted plates. It is equipped
with a vacuum station which may be used for protocols requiring
liquid drawn through a column as commonly found in many purification
protocols.
Return to top
For pipetting 384 well RT-PCR plates, Pennington employees
may schedule an appointment for the instrument (R_GCF_Rosie)
on the Outlook calendar at least 24 hours in advance.
Allow one hour for each plate you wish to pipet.
Detailed instructions for using the Outlook calendar to schedule an instrument (resource) may be found in the Scheduling Resources - Frequently Asked Questions.
For scheduling less than 24 hours in advance or for customers
outside of Pennington please contact
GCF
for an appointment.
Return to top
Cost for Robot Pipetting of a 384 well RT-PCR plate
| Plate over half-full | $ 100.00 |
| Plate less than half-full | $ 50.00 |
| Cancelled appointment less than 24 hours in advance |
$ 25.00 |
| Template only | $ 50.00 |
| Master Mix only | $ 50.00 |
Cost to hand-pipette a full 384 well RT-PCR plate
| Description | Unit Cost |
Extended Cost |
| Filter Pipet Tips | $ 6.75 | $ 40.50 |
| 384 well plate | $ 4.14 | $ 4.14 |
| plate seal tape | $ 1.45 | $ 1.45 |
| Labor | $ 25.00 | $ 50.00 |
| Total | $ 96.09 |
Return to top
The MultiProbe II robot is programmed to pipet 10ul reactions
into an Applied Biosystems 384 well Optical plate. Save the spreadsheet
Robotic RT-PCR Calculator.xls
to your computer to calculate the correct amount of template and master mix
for your experiment. It is extremely important that you use properly
calibrated pipettors for RT-PCR experiments. The spreadsheet provided
calculates the correct amount of reagents needed, but if your pipettors
are off, you may find that you haven’t provided the proper amounts of
each reagent. QPCR is quantitative. Your data accuracy is highly
dependent upon the accuracy of your tools.
The following must be supplied to the Genomics Core Facility:
- RNA or cDNA template
- The template should be provided in an Applied Biosystems
96 well optical plate. The total amount of template added
is proportionally scaled down from the amount used in your
manual reactions. If you normally use 30ul total reaction
volume, you are scaling down to 10ul; therefore, divide the
quantity of template by 3.
- The robot pipets 3ul of your template into each reaction.
You will need to adjust your template concentration to ensure
that the amount of template you wish to put into your reaction
is contained in 3ul. (For example, in a manually set up reaction
you normally use 2ul of a 2ng/ul concentration template for a
total of 4ng template in a 10ul reaction. The new concentration
needed for the robot is 1.33ng/ul giving the same 4ng total in
your robotically pipetted reaction mix.) You may make template
master plates that can be used to set up multiple 384 well plates
on the robot. The plates are sealed and stored at –70C between runs.
- The spreadsheet includes 10ul excess template in each well to insure
that the robot pipet tip is able to pick up template for all wells
in your experiment.
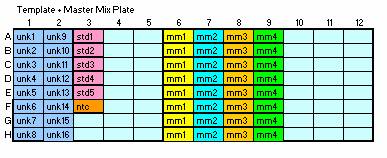
- For duplicate reactions on the 384 well plate, your templates
should be placed in column order on a half-skirted
96 well plate (A1, B1, C1….H1). Do not skip any wells.
Place your standards and NTC last.

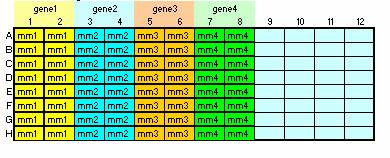
For triplicate reactions on the 384 well plate, your templates
should be placed in double columns as shown below
(A1, A2, B1, B2, …., H1, H2, A3, A4, …..H3, H4….). Do not skip any wells.
Place your standards and NTC last.

- Master Mix
The master mix contains all of your reagents except the template. The robot
will pipet 7ul per well into the 384 well plate. Place each master mix into
eight vertical wells (a column) of a 96well plate. The spreadsheet
(Robotic RT-PCR Calculator) will indicate the correct volume for each well
for duplicate or triplicate reactions.

OR
OR for large amounts of Master Mix
Divide the master mix volume for each well as determined on the Robotic
RT-PCR Calculator spreadsheet equally between two adjacent horizontal wells.
- 384 Well Plate Layout
The robot pipets into the plate in column order. Your 384 well plate
will be laid out in that order (A1, B1, C1,…., P1). For duplicate reactions
the robot will pipet from well A1 in your template plate to wells A1 and B1
in your 384 well reaction plate.

Triplicate reactions will be placed in A1, A2, A3, B1, B2, B3, C1,
C2, C3…..on your 384 well plate.

- Supplies
The Genomics Core Facility will provide robotic pipet tips, 384 well
PCR plates, and plate sealing tape.
- Submission to the Genomics Core Facility
The following must be provided to the Core Laboratory 24 hours prior
to your scheduled run on the 7900:
- Total number of samples (include standards and no template control)
- Number of genes to be tested on the plate
- Number of replicates
- Column location of each master mix in a 96 well plate. Please
indicate whether the master mix is included in your template plate
or in a separate plate.
Alternatively, you may provide an Excel plate map of each plate
containing samples and master mixes.
- You must be present at the end of the robotic pipetting
to remove your plate, check it for pipetting errors, seal it,
centrifuge it, and start your run on the 7900.
-
When 384 well plates are pipetted by robot, the samples are in
vertical well order. To ease data entry for these plates refer to the
document
Using Excel to Set Up a Real-Time PCR Plate
Return to top